How Many Electrons Does Helium Have in Its Outer Shell
Helium is located in period 1 group 18 of the Periodic Table and has an atomic number equal to 2. 3 How many total electrons and valence electrons are found in an atom of lithium.
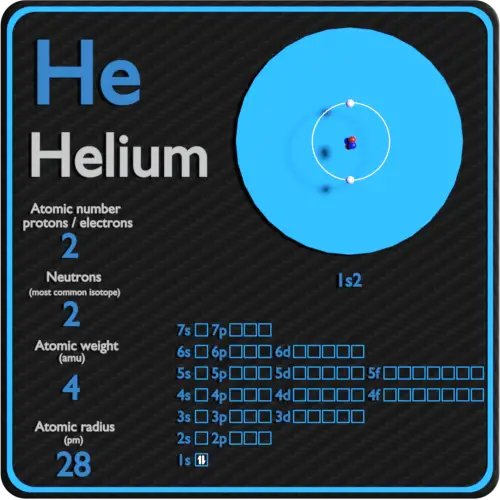
Helium Periodic Table And Atomic Properties
9 How many 4s electrons are in GE.
. They each have two electrons in their outer shell. As a result neutral helium will only have 2 electrons surrounding its nucleus. The atom helium has only 2 electrons and assuming it is neutral and 2 protons and neutrons.
Helium does have a full outer shell of Electrons its two electrons occupy the 1s shell. Helium atoms have two electrons in their outer shell and the other noble gases have eight electrons in their outer shell. The greater distance between the electrons in the outer shells and the protons in the nucleus mean the outer shell electrons experience less of a force of attraction to the nucleus than.
1 Lithium Has An Atomic Number Of 3. Silver has 47 electrons per atom and its molar mass is 107. Of these inert elements the helium atom has 2 electrons in its outermost shell and all other elements have atoms with 8 electrons in the outermost shell.
Helium has only two electrons in its outermost shell. Notice that chemists normally put the number and then the charge so 2- not -2. How Many Electrons Does Helium Have In Its Outer Shell.
Group 18 elements helium neon and argon are shown in Figure 2 have a full outer or valence shell. Helium atom has 2 electrons in its valence shell but its valency is. 8 Why does C have 4 valence electrons.
Which means its easier to gain 1 electron to form a full outer shell to make it a ion. It is very stable with only two electrons in its external orbital Shell of Valenza. Helium atoms have two electrons in their outer shell and the other noble gases have eight electrons in their outer shell.
Although it has only two electrons it is still grouped with the noble gases they have Electrons in their most external orbitals. How many electrons are needed to fill heliums. How Many Electrons Does Helium Have In Its Outer Shell The octet rule refers to the tendency of atoms to prefer to have eight electrons in the valence shell outer orbital.
Two electrons in its outermost and only shell two valence electrons. 7 What family has 8 valence electrons. 4 What is the valence electron for lithium.
Helium atoms have two electrons in their outer shell and the other noble gases have eight electrons in their outer shell. 6 How do I find valence electrons. Forbes best undergraduate business schools 2022.
2 How many valence electrons does lithium have in its outer shell. How Many Electrons Are There In The Outermost valence Shell. Each atom is made up of a nucleus and a number of electrons that orbit the nucleus.
That means there are 2 electrons in a helium atom. Atoms can have this many electrons but they do not have to have this many electrons in each shell. It was observed that the atoms of elementshaving a completely filled outermost shell show little chemical activity.
11 How many 4p electrons are in GE. How many electrons does tin have. Of these inert elements the helium atom has 2 electrons in its outermost shell and all other elements have atoms with 8 electrons in the outermost shell.
The energy required to remove one of them is the highest ionization energy of any atom in the periodic table. Helium has two electrons in total and according to the aufbau principle it adopts the electronic configuration 1 s 2. The electrons of the outermost energy level determine the energetic stability of the atom and its Bohr diagrams indicate how many electrons fill each principal shell.
How many electrons does helium have is one of the most frequently asked questions. 4 Does GE have 4 valence electrons. Helium atoms have two electrons in their outer shell and the other noble gases have eight electrons in their outer shell.
10 How many energy levels and valence electrons does an atom of germanium Ge have. 5 What is the valence shell of GE. Posted on April 23 2022 by.
Intown suites newport news va. Similarly neon has a complete outer 2n shell containing eight electrons. They all have a single electron in their outer shell.
It has 30 electrons in its outer shell. For instance Sodium Na resides in Period 3 Group 1 which implies that it has 3 shells and a single electron in its valence shell. How Many Electrons Does Helium Have In Its Outer Shell The octet rule refers to the tendency of atoms to prefer to have eight electrons in the valence shell outer orbital.
Why does helium only have 2 valence electrons. The first shell can hold 2 electrons maximum which is what Helium has in its outer shell. In other words their combining capacity is 0.
5 How many vacancies are there in the outermost shell of lithium.
Chem4kids Com Helium Orbital And Bonding Info
No comments for "How Many Electrons Does Helium Have in Its Outer Shell"
Post a Comment